Catalogue
Mouse anti Vimentin
Catalog number: MUB1900P| Clone | RV202 |
| Isotype | IgG1 |
| Product Type |
Monoclonal Antibody Primary Antibodies |
| Units | 0.1 mg |
| Host | Mouse |
| Species Reactivity |
Canine Caprine Chicken Hamster Human Monkey Mouse Rat Swine Xenopus Zebrafish |
| Application |
Flow Cytometry Immunocytochemistry Immunohistochemistry (frozen) Immunohistochemistry (paraffin) Western Blotting |
Background
Vimentin (57 kDa) is the intermediate filament protein (IFP) of mesenchymal cells. This IFP however often deviates from the tissue-specific and developmentally regulated pattern of expression. Besides its typical expression in most cultured cells, vimentin is also expressed together with several other IFPs during early stages of development. As differentiation proceeds, vimentin is exchanged for the tissue-specific intermediate filament type. Also in cancers, vimentin is often expressed in addition to the tissue-specific IFP.
Product
Each vial contains 100 ul 1 mg/ml purified monoclonal antibody in PBS containing 0.09% sodium azide.
Purification Method: ProtG affinity purification
Concentration: 1 mg/mL
Specificity
RV202 reacts exclusively with vimentin, which is expressed in mesenchymal cells and mesenchymal derived tumors e.g. lymphoma, sarcoma and melanoma.
Species Reactivity: Pieper et al (European Journal of Biochemistry 1992; included) show in figure 5 and 6 that replacement of exons 7-9 in the vimentin gene (construct Vvim2) results in a vimentin protein that is no longer detected by RV202, indicating that the epitope for RV202 is located in the C-terminal region of vimentin, comprising amino acid range 409-466 . RV202 has a very broad species cross-reactivity, ranging from human to Xenopus, and must therefore recognize an evolutionary conserved amino acid sequence.
Applications
RV202 is suitable for immunoblotting, immunocytochemistry, immunohistochemistry on frozen and paraffin embedded tissues and flow cytometry. Optimal antibody dilutions should be determined by titration; recommended range is 1:100 – 1:200 for flow cytometry, and for immunohistochemistry with avidin-biotinylated Horseradish peroxidase complex (ABC) as detection reagent, and 1:100 – 1:1000 for immunoblotting applications.
Storage
The antibody is shipped at ambient temperature and may be stored at +4°C. For prolonged storage prepare appropriate aliquots and store at or below -20°C. Prior to use, an aliquot is thawed slowly in the dark at ambient temperature, spun down again and used to prepare working dilutions by adding sterile phosphate buffered saline (PBS, pH 7.2). Repeated thawing and freezing should be avoided. Working dilutions should be stored at +4°C, not refrozen, and preferably used the same day. If a slight precipitation occurs upon storage, this should be removed by centrifugation. It will not affect the performance or the concentration of the product.
Shipping Conditions: Ship at ambient temperature.
Caution
This product is intended FOR RESEARCH USE ONLY, and FOR TESTS IN VITRO, not for use in diagnostic or therapeutic procedures involving humans or animals. It may contain hazardous ingredients. Please refer to the Safety Data Sheets (SDS) for additional information and proper handling procedures. Dispose product remainders according to local regulations.This datasheet is as accurate as reasonably achievable, but Nordic-MUbio accepts no liability for any inaccuracies or omissions in this information.
References
1. Ramaekers, F., Huysmans, A., Schaart, G., Moesker, O., and Vooijs, P. (1987). Tissue distribution of Keratin 7 as monitored by a monoclonal antibody, Exp Cell Res 170, 235-49.
2. Viebahn, C., Lane, E. B., and Ramaekers, F. C. (1988). Keratin and vimentin expression in early organogenesis of the rabbit embryo, Cell Tissue Res 253, 553-62.
3. Pieper, F. R., Schaart, G., Krimpenfort, P. J., Henderik, J. B., Moshage, H. J., van de Kemp, A., Ramaekers, F. C., Berns, A., and Bloemendal, H. (1989). Transgenic expression of the muscle-specific intermediate filament protein desmin in nonmuscle cells, J Cell Biol 108, 1009-24.
4. Raats, J. M., Pieper, F. R., Vree Egberts, W. T., Verrijp, K. N., Ramaekers, F. C., and Bloemendal, H. (1990). Assembly of amino-terminally deleted desmin in vimentin-free cells, J Cell Biol 111, 1971-85.
5. Ramaekers, F., van Niekerk, C., Poels, L., Schaafsma, E., Huijsmans, A., Robben, H., Schaart, G., and Vooijs, P. (1990). Use of monoclonal antibodies to keratin 7 in the differential diagnosis of adenocarcinomas, Am J Pathol 136, 641-55.
6. van Beijnum JR, Huijbers EJM, van Loon K, Blanas A, Akbari P, Roos A, Wong TJ, Denisov SS, Hackeng TM, Jimenez CR, Nowak-Sliwinska P, Griffioen AW. Extracellular vimentin mimics VEGF and is a target for anti-angiogenic immunotherapy. Nat Commun. 2022 May 23;13(1):2842. doi: 10.1038/s41467-022-30063-7.
Safety Datasheet(s) for this product:
| NM_Sodium Azide |

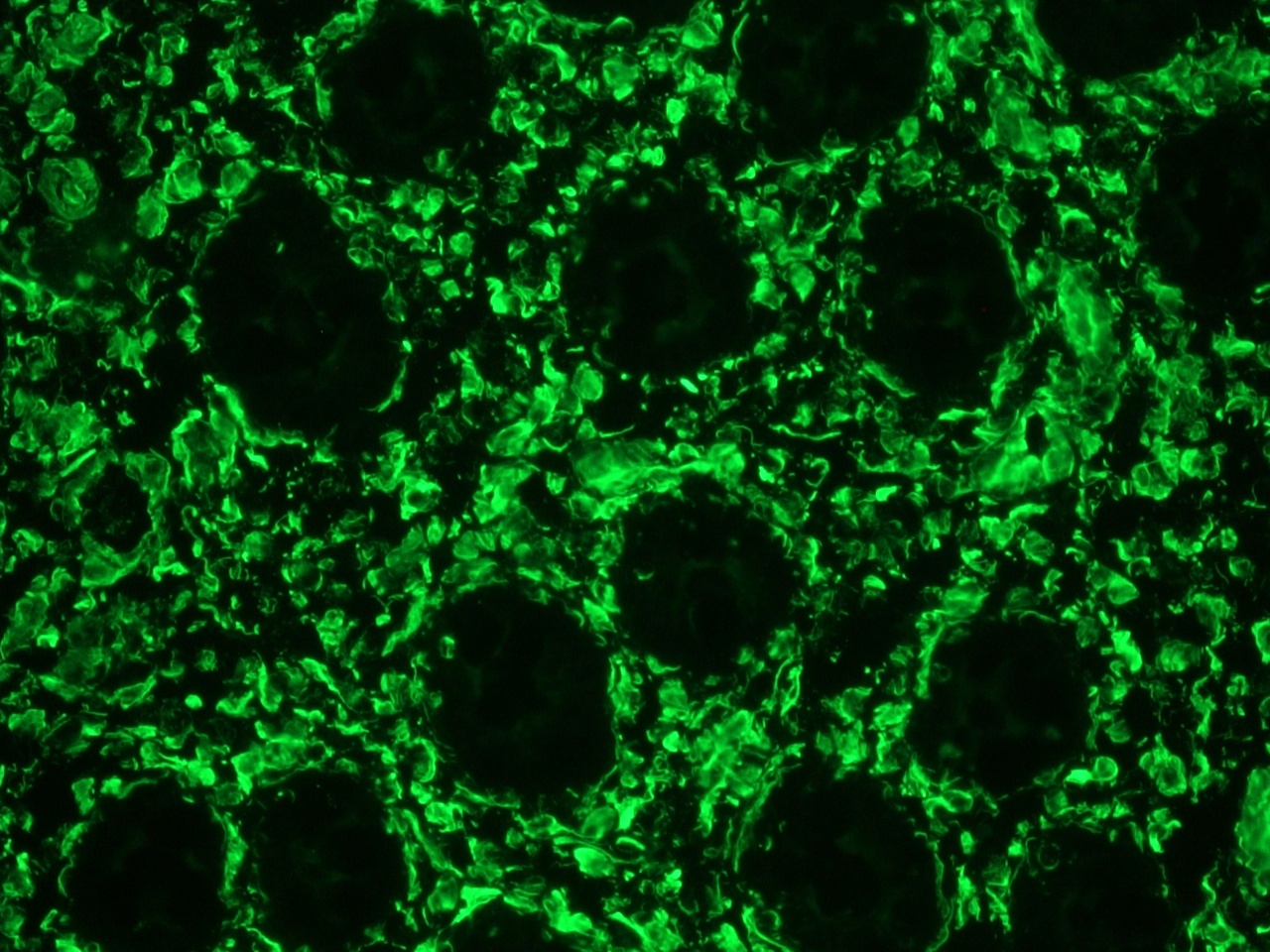
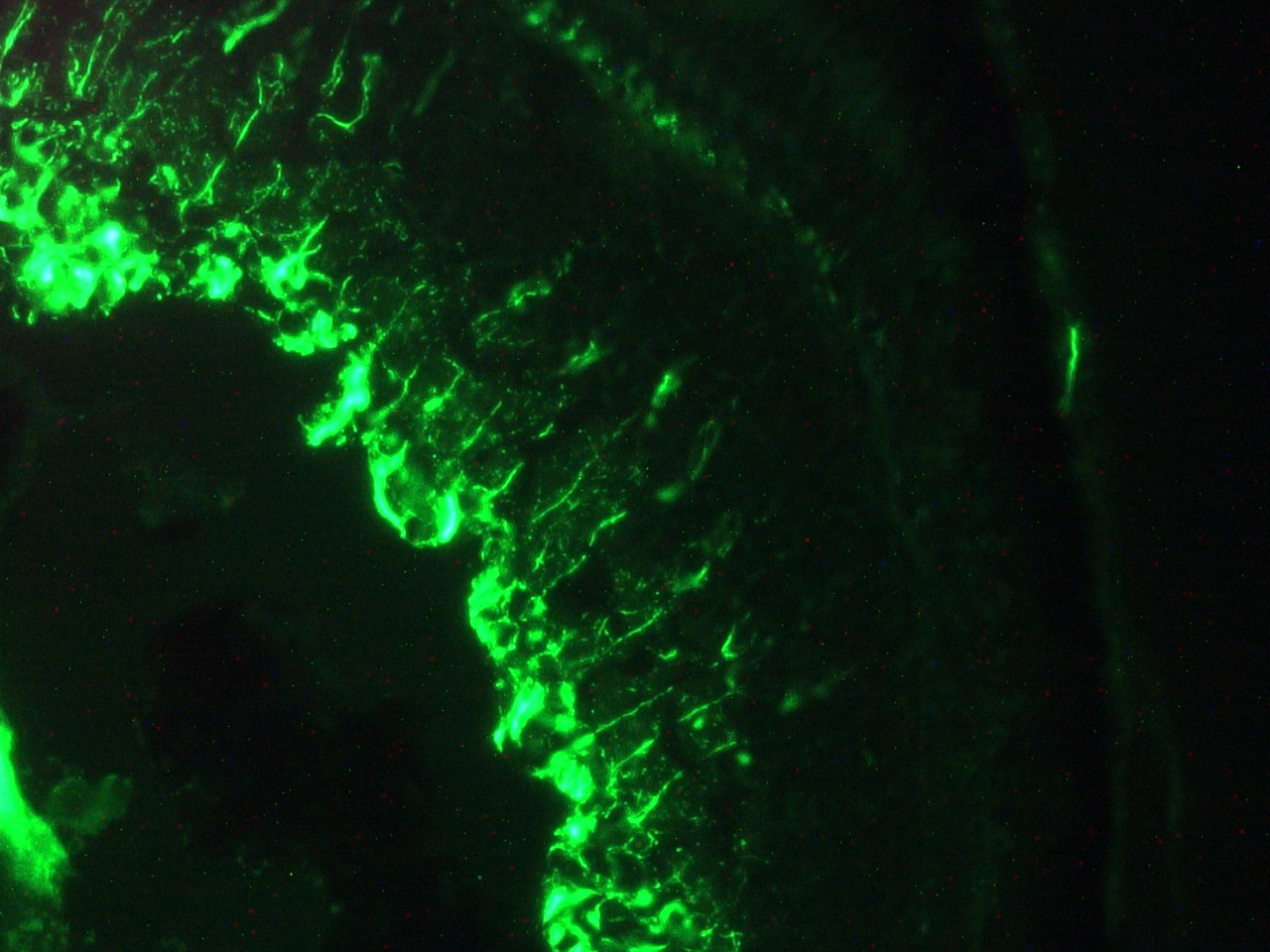
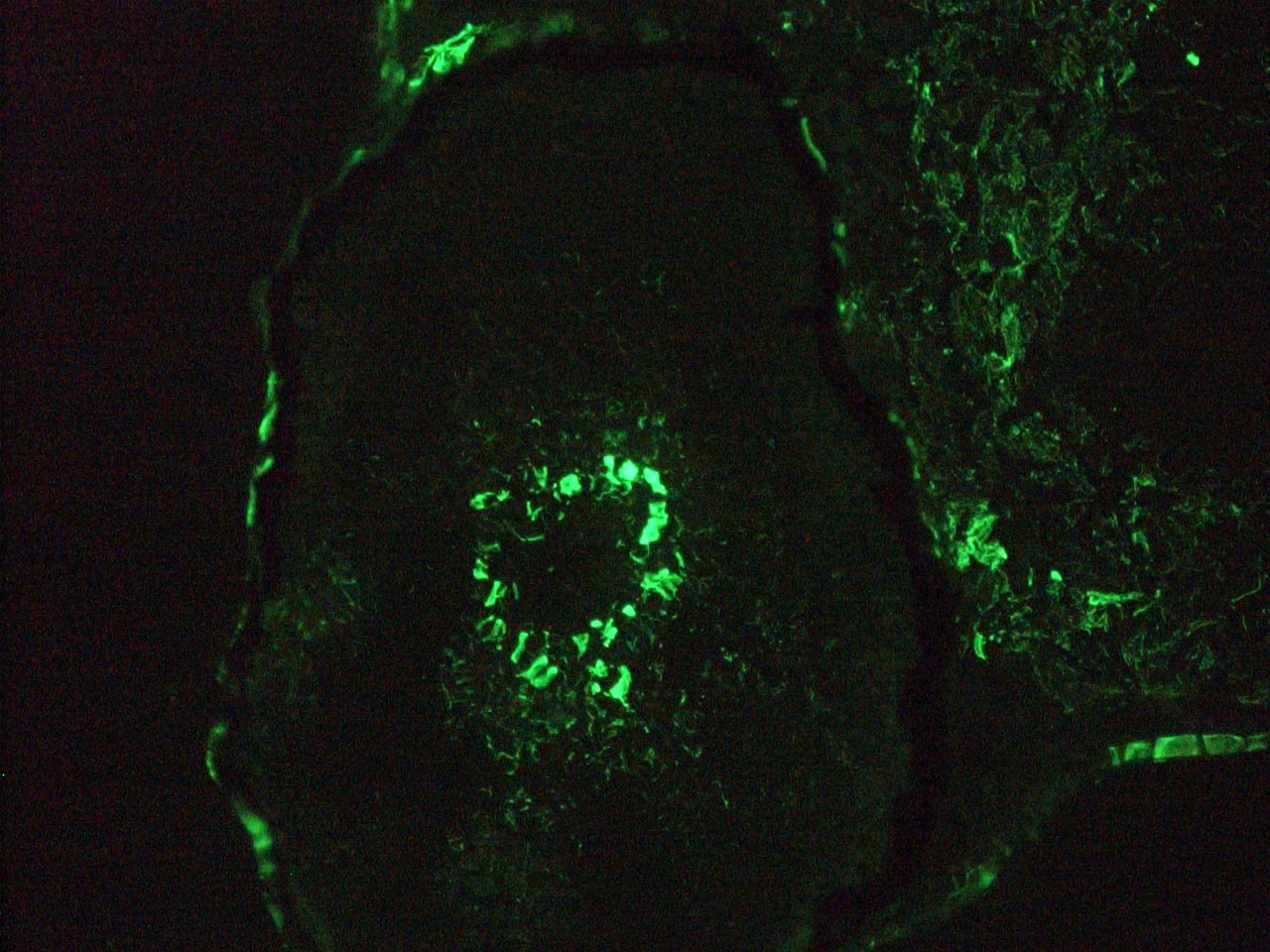
Figure 1. Immunohistochemistry on frozen section of swine colon showing positive staining in connective tissue cells and no reactivity in epithelial cells.
Figure 2. Western blotting result showing the specific reactivity of MUB1900P with the 57kDa protein band of vimentin in both the mouse (3T3 mouse fibroblasts; left lane) and human (normal human dermal fibroblasts; right lane) cell extracts.

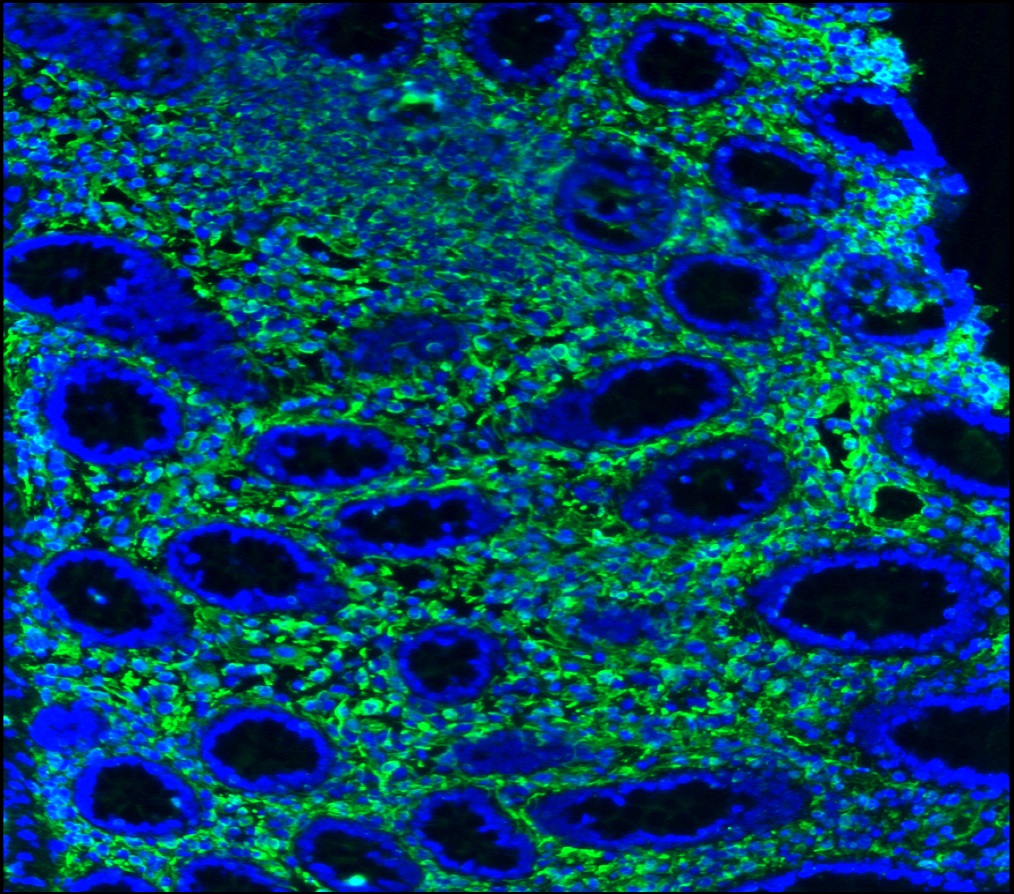
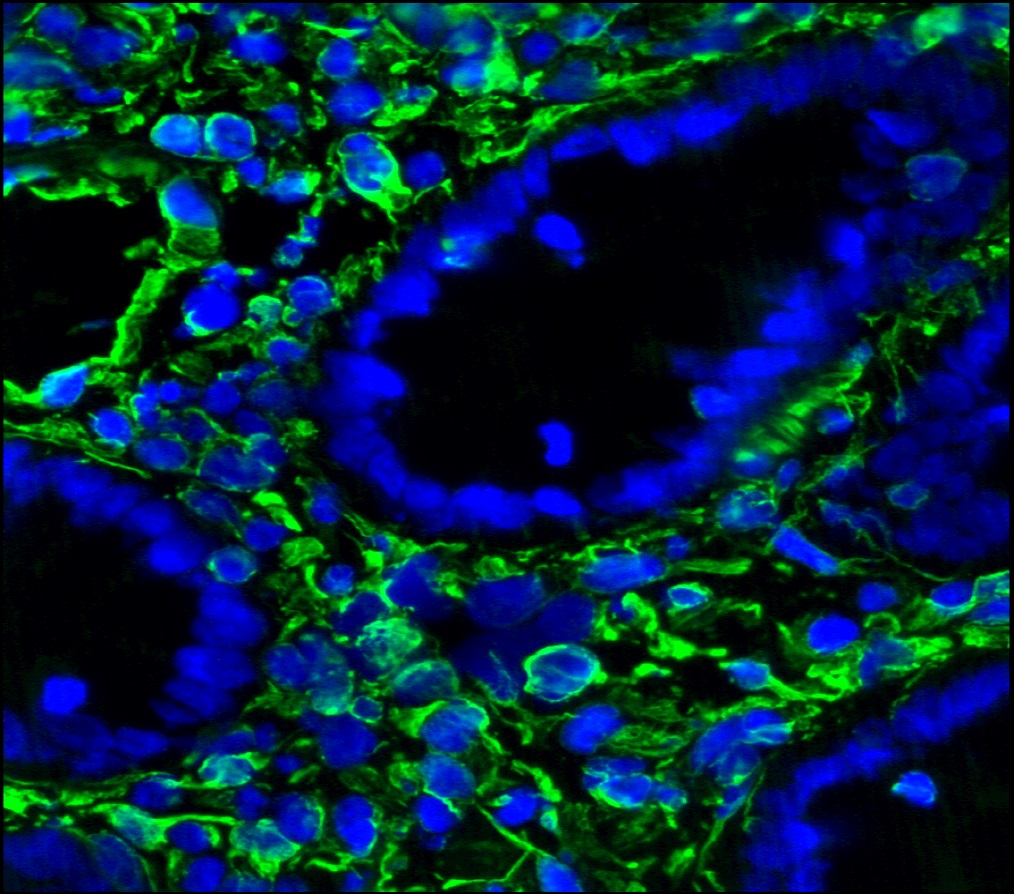
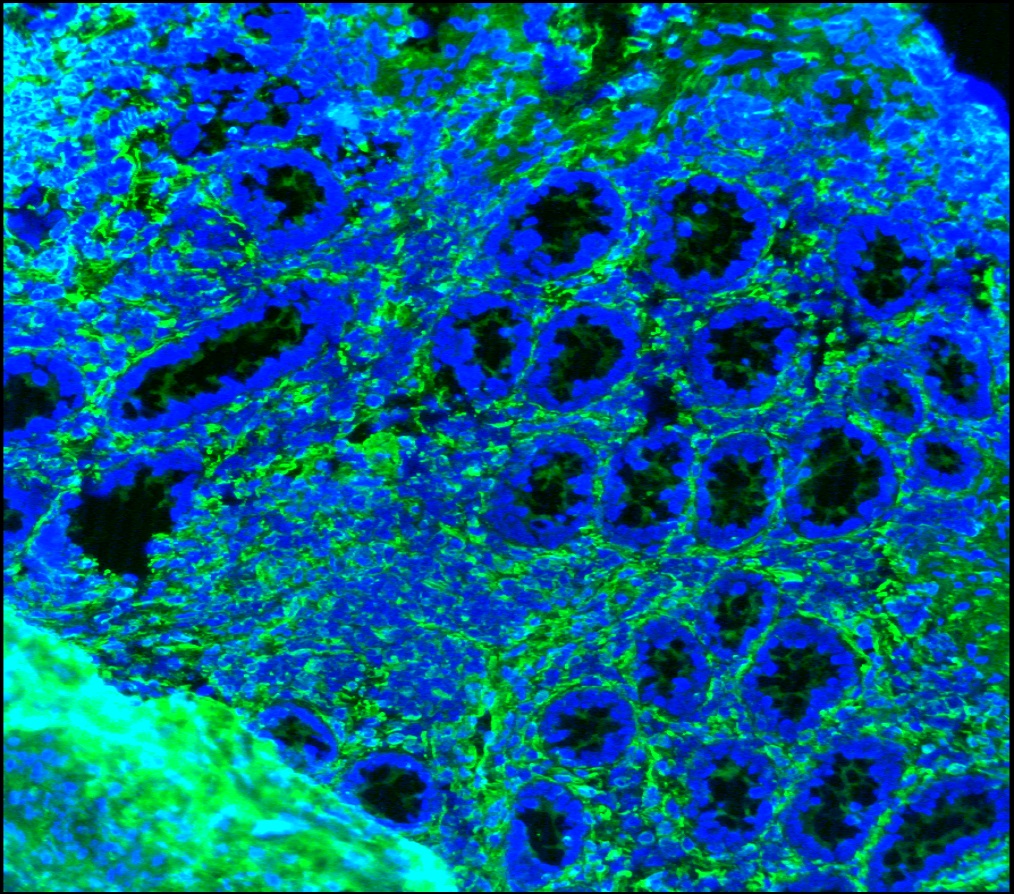
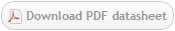
Figure 3. Immunohistochemistry on frozen section of swine colon showing positive staining in connective tissue cells and no reactivity in epithelial cells. Nuclear staining with DAPI.

Figure 4. Immunohistochemistry on frozen section of swine colon showing positive staining in connective tissue cells and no reactivity in epithelial cells. Nuclear staning with DAPI.

Figure 5. Immunohistochemistry on frozen section of swine colon showing positive staining in connective tissue cells and no reactivity in epithelial cells. Nuclear staning with DAPI.

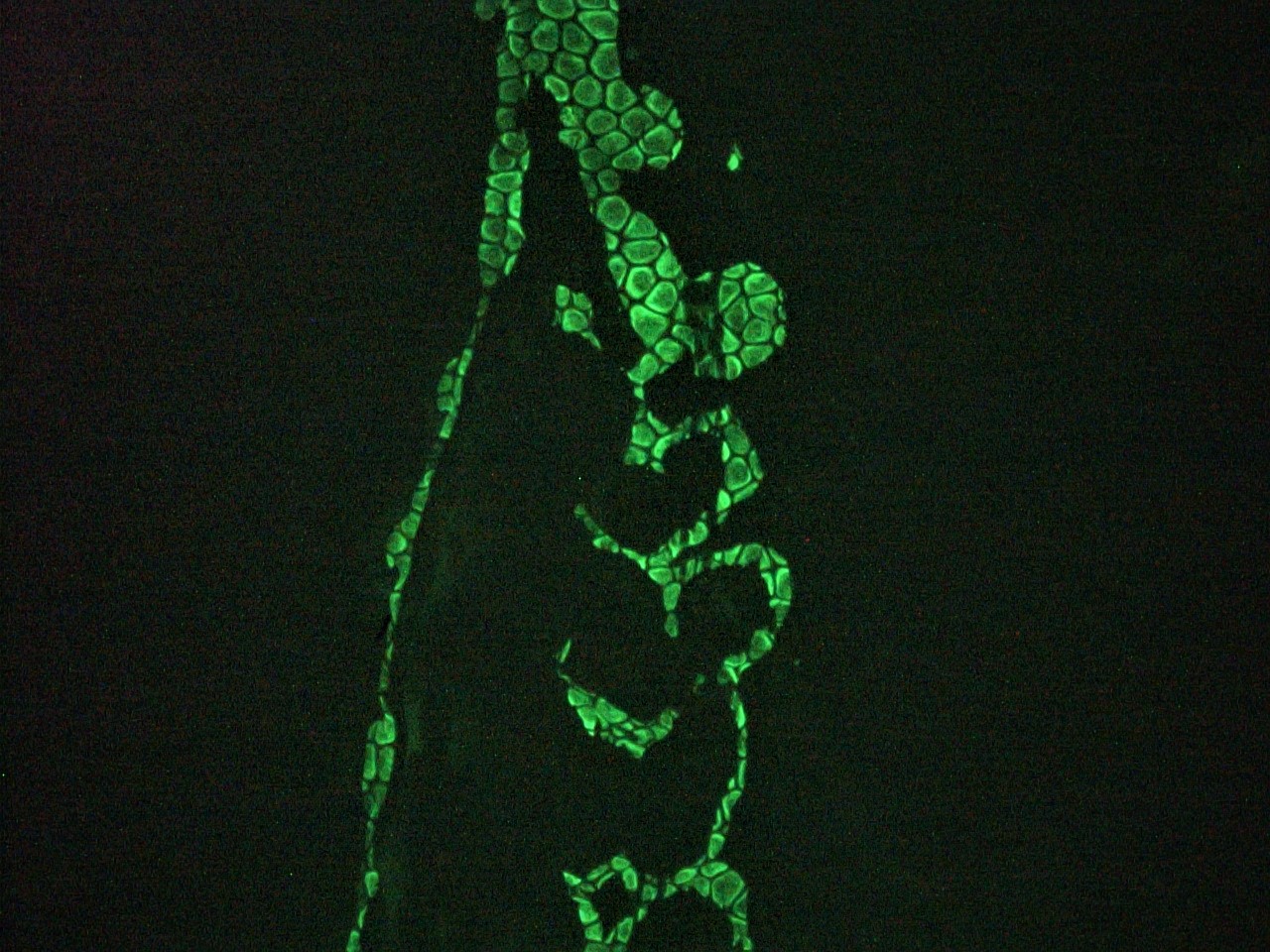
Figure 6. Immunohistochemistry on frozen section of zebra fish embryo.

Figure 7. Immunohistochemistry on frozen section of zebra fish embryo

Figure 8. Immunohistochemistry on frozen section of zebra fish embryo

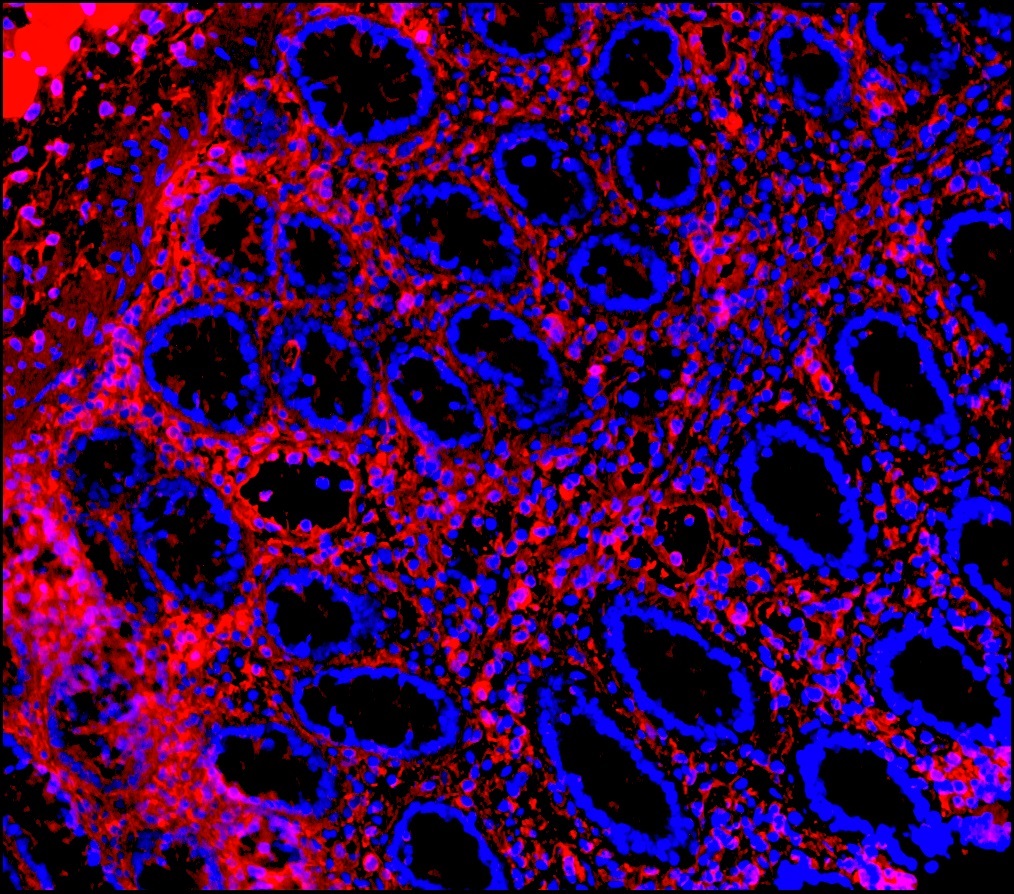
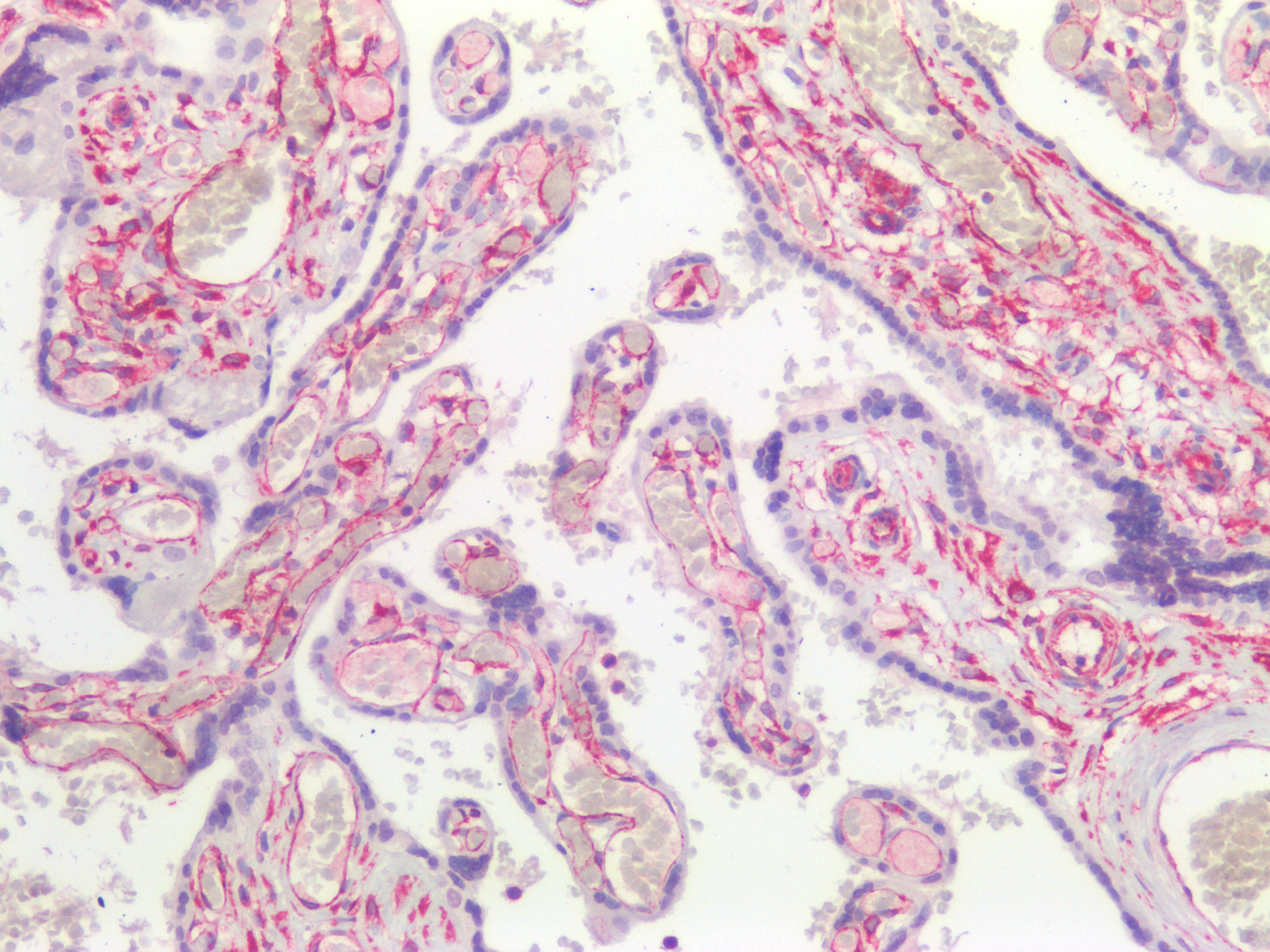
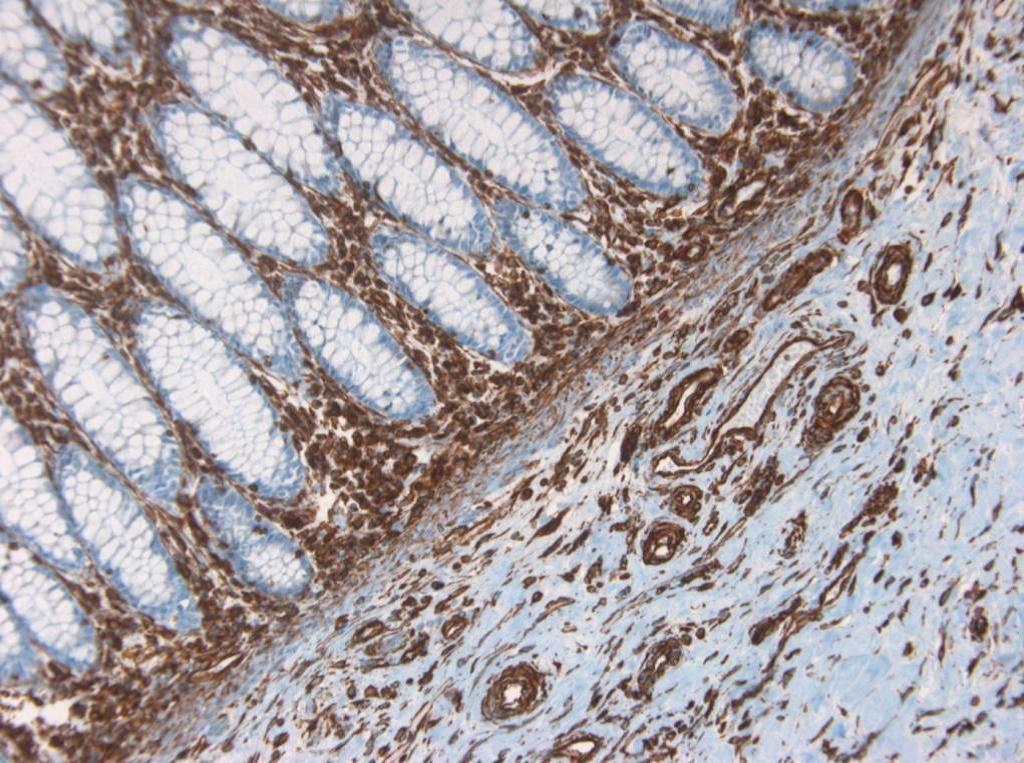
Figure 9. Immunohistochemistry on formalin fixed, paraffin embedded section of human placenta showing positive staining in connective tissue cells and no reactivity in epithelial cells.

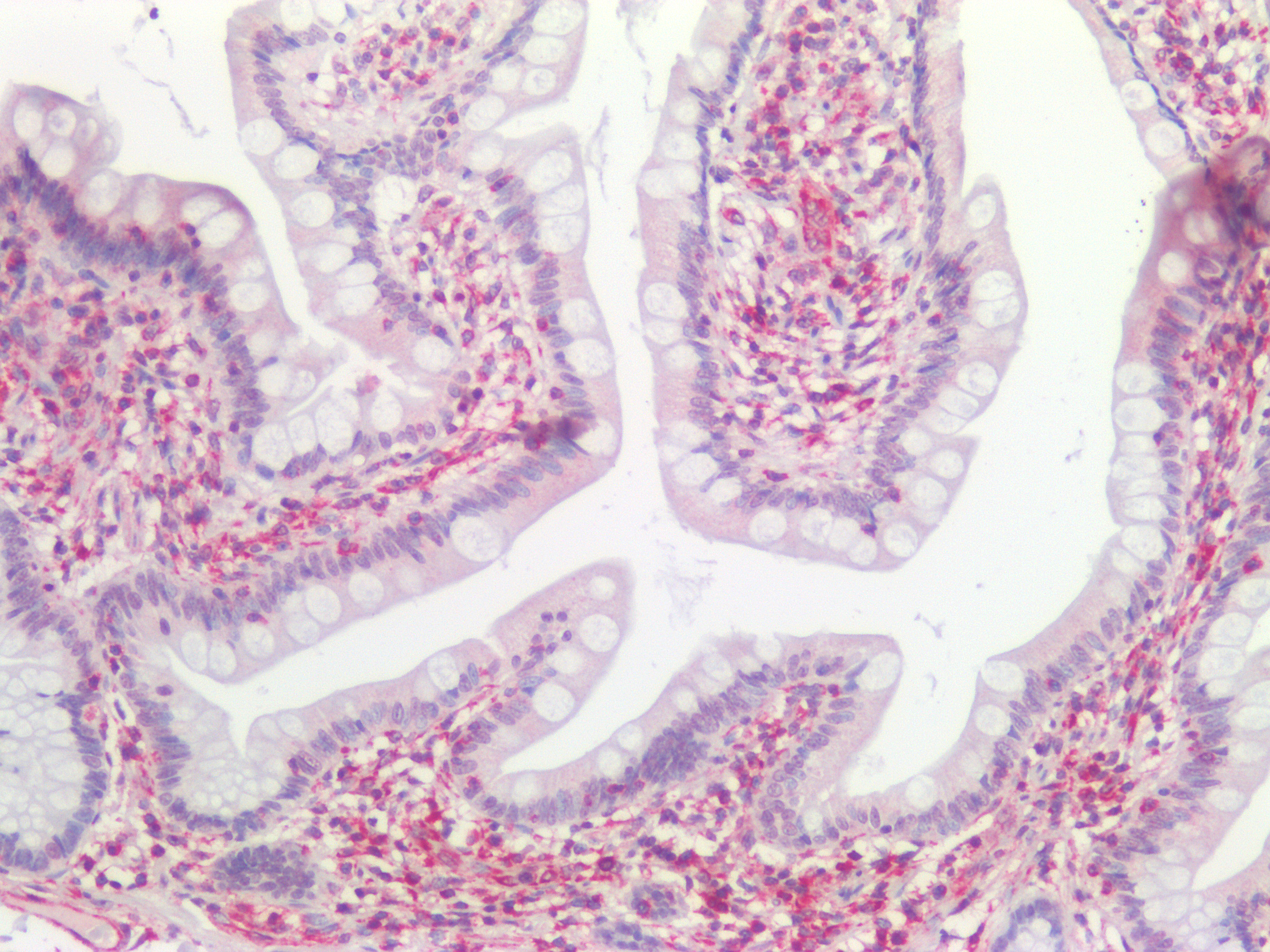
Figure 10. Immunohistochemistry on formalin fixed, paraffin embedded section of human small intestine showing positive staining in connective tissue cells and no reactivity in epithelial cells.

Figure 11. Immunohistochemistry on formalin fixed, paraffin embedded section of human small intestine showing positive staining in connective tissue cells and no reactivity in epithelial cells.

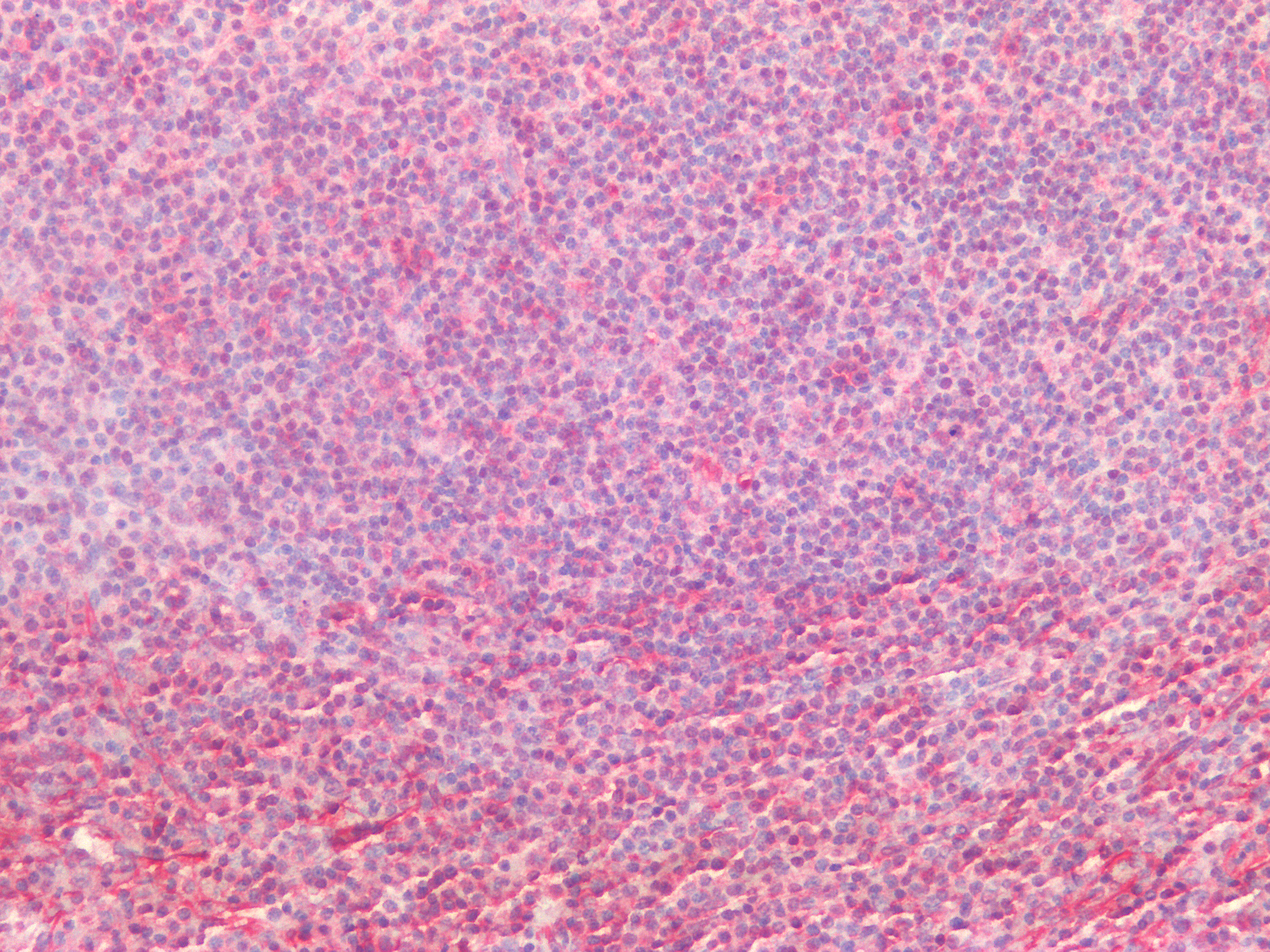
Figure 12. Immunohistochemistry on formalin fixed, paraffin embedded section of human spleen showing positive staining in connective tissue cells and lymphoid cells.

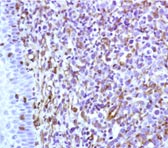
Figure 13. Immunohistochemistry on formalin fixed, paraffin embedded section of human tonsillar lymphoma.

Figure 14. Immunohistochemistry on frozen section of swine colon showing positive staining in connective tissue cells and no reactivity in epithelial cells. Nuclear staning with DAPI.