Catalogue
Mouse anti Cardiotin
Catalog number: MUB0307P| Clone | R2G |
| Isotype | IgM |
| Product Type |
Primary Antibodies |
| Units | 0.1 ml |
| Host | Mouse |
| Species Reactivity |
Canine Caprine Feline Hamster Human Monkey Mouse Rabbit Rat Xenopus Zebrafish |
| Application |
Immunohistochemistry (frozen) Immunohistochemistry (paraffin) Western Blotting |
Background
Cardiotin is a high molecular weight protein complex (300 kDa) loCated in the mitochondria of cardiomyocytes and skeletal muscle. The cardiotin structure exists of subunits of 60 kDa and 100 kDa, probably in a tetrameric configuRation. Both subunits contain the same amino-terminal 14 amino-acid sequence, showing high homology to Human skeletal muscle α-actinin. During cardiac contractile dysfunction and myocard cell differentiation, the cardiotin distribution is affected. Compared to other structural proteins, cardiotin is one of the first to respond to insults (ischemia, fibrillation) that influence the functional status of cardiomyocytes.
Source
R2G is a mouse monoclonal IgM antibody derived by fusion of SP2/0-Ag14 mouse myeloma cells, with spleen cells from a mouse immunized with a total protein extract of chicken gizzard.
Product
Each vial contains 0.1 ml monoclonal antibody in PBS containing 0.09% sodium azide. The antibody is purified by ammonium sulphate precipitation and will threfore contains fetal calf serum derived protein contaminants
Purification Method: Ammonium sulphate precipitation
Concentration: 1 mg/ml
Specificity
R2G reacts with cardiotin, a mitochondrion-associated protein, which is present in cardiomyocytes and skeletal muscle.
Applications
R2G is useful for immunohistochemistry on frozen and paraffin-embedded tissue and immunoblotting. In immunoblotting assays R2G reacts with the 300 kDa cardiotin protein complex and its 100 kDa and 60 kDa subunits. Recommended range is 1:25 – 1:100 for immunohistochemistry with avidin-biotinylated Horseradish peroxidase complex (ABC) as detection reagent. Optimal antibody dilution for immunoblotting applications should be determined by titration.
Storage
The antibody is shipped at ambient temperature and may be stored at +4°C. For prolonged storage prepare appropriate aliquots and store at or below -20°C. Prior to use, an aliquot is thawed slowly in the dark at ambient temperature, spun down again and used to prepare working dilutions by adding sterile phosphate buffered saline (PBS, pH 7.2). Repeated thawing and freezing should be avoided. Working dilutions should be stored at +4°C, not refrozen, and preferably used the same day. If a slight precipitation occurs upon storage, this should be removed by centrifugation. It will not affect the performance or the concentration of the product.
Shipping Conditions: Ship at ambient temperature.
Caution
This product is intended FOR RESEARCH USE ONLY, and FOR TESTS IN VITRO, not for use in diagnostic or therapeutic procedures involving humans or animals. It may contain hazardous ingredients. Please refer to the Safety Data Sheets (SDS) for additional information and proper handling procedures. Dispose product remainders according to local regulations.This datasheet is as accurate as reasonably achievable, but Nordic-MUbio accepts no liability for any inaccuracies or omissions in this information.
References
1. Schaart, G., van der Ven, P. F., and Ramaekers, F. C. (1993). Characterization of cardiotin, a structural component in the myocard, Eur J Cell Biol 62, 34-48.
2. Schaart, G., Moens, L., Endert, J. M., and Ramaekers, F. C. (1997). Biochemical characterization of cardiotin, a sarcoplasmic reticulum associated protein, FEBS Lett 403, 168-72.
3. Ausma, J., Wijffels, M., van Eys, G., Koide, M., Ramaekers, F., Allessie, M., and Borgers, M. (1997). Dedifferentiation of atrial cardiomyocytes as a result of chronic atrial fibrillation, Am J Pathol 151, 985-97.
4. Dispersyn, G. D., Geuens, E., Ver Donck, L., Ramaekers, F. C., and Borgers, M. (2001). Adult Rabbit cardiomyocytes undergo hibernation-like dedifferentiation when co-cultured with cardiac fibroblasts, Cardiovasc Res 51, 230-40.
5. Ausma, J., Litjens, N., Lenders, M-H., Duimel, H., Mast, F., Wouters, L., Ramaekers, F., Allessie, M., and Borgers, M. (2001). Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat, J Mol Cell Cardiol 33, 2083-94.
6. Dispersyn, G. D., Mesotten, L., Meuris, B., Maes, A., Mortelmans, L., Flameng, W., Ramaekers, F. C., and Borgers, M. (2002). Dissociation of cardiomyocyte apoptosis and dedifferentiation in infarct border zones, Eur Heart J in press.
7. Ausma, J., van der Velden, H. M., Lenders, M. H., van Ankeren, E. P., Jongsma, H. J., Ramaekers, F. C., Borgers, M., and Allessie, M. A. (2003). Reverse structural and gap-junctional remodeling after prolonged atrial fibrillation in the goat. Circulation 107, 2051-2058.
8. RR Pochampally, BT Neville, EJ Schwarz, MM Li, DJ Prockop (2004). Rat adult stem cells (marrow stromal cells) engraft and differentiate in chick embryos without evidence of cell fusion, Proc Natl Acad Sci USA 101, 9282-85
Protein Reference(s)
Database Name: UniProt
Accession Number: multiple
Safety Datasheet(s) for this product:
| NM_Sodium Azide |

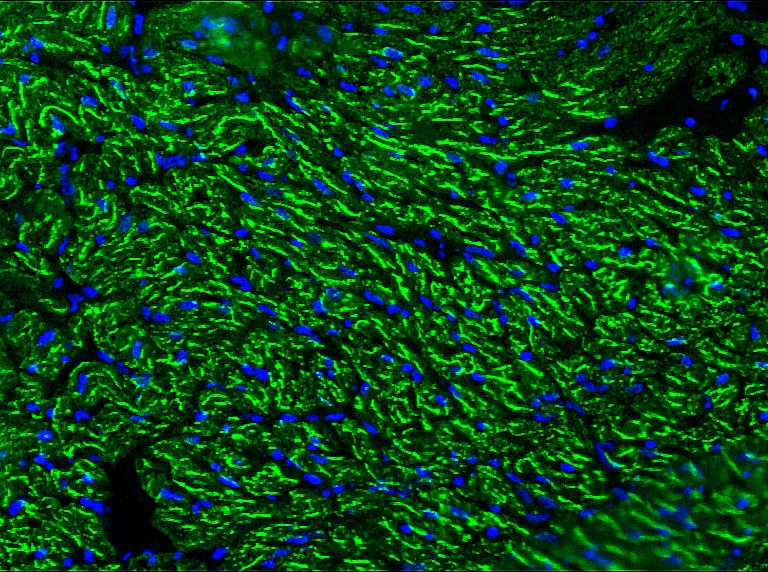
Figure 1. Indirect immunofluorescence staining of swine heart with MUB0307P (R2G) showing positive staining of mitochondria in cardiomyocytes.

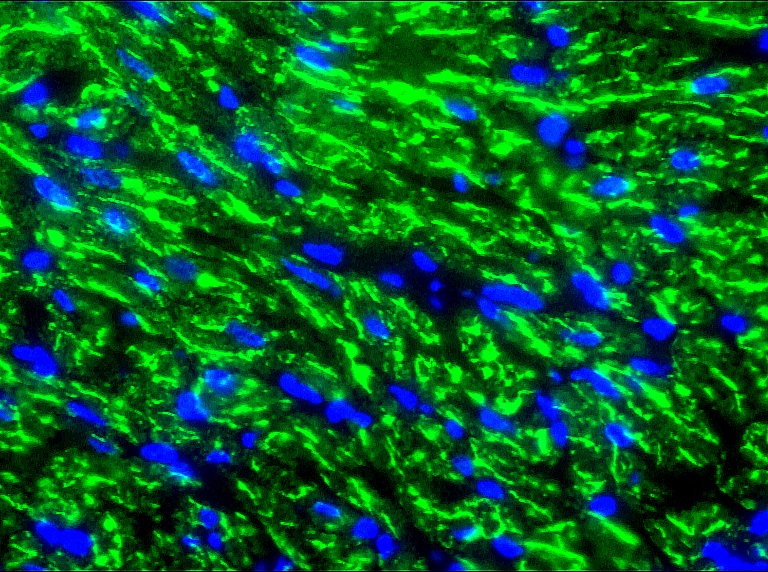
Figure 2. Indirect immunofluorescence staining of swine heart with MUB0307P (R2G) showing positive staining of mitochondria in cardiomyocytes.

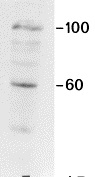
Figure 3. Immunoblotting result of MUB0307P (R2G) on a mitochondrion isolation from swine heart