Catalogue
Mouse anti actin alpha-smooth muscle
Catalog number: MUB0100P| Clone | 1A4 |
| Isotype | IgG2a |
| Product Type |
Primary Antibodies |
| Units | 0.05 mg |
| Host | Mouse |
| Species Reactivity |
Caprine Chicken Human Monkey Quail Rat Sheep Swine Xenopus |
| Application |
Electron microscopy Immunocytochemistry Immunohistochemistry (frozen) Immunohistochemistry (paraffin) Western Blotting |
Background
Among the six actin isoforms described in mammals, two are found in virtually all cells (β- and γ-cytoplasmic), two are detected in smooth muscle cells (α- and γ-smooth muscle) and two are present in striated muscles, one predominantly in skeletal (α-skeletal) and one in cardiac (α-cardiac) muscle cells. These actin isoforms differ slightly in their N-terminus, but the sequence of each of these actins is highly conserved in higher vertebRates. Alpha-smooth muscle actin is abundant in vascular and visceral smooth muscle cells. In addition, it has also been shown to appear in stress fibers of fibroblastic cells during pathological situations involving contractile phenomena such as wound healing and fibrocontractive diseases.
Source
α-SM1 (clone 1A4) is a Mouse monoclonal IgG2a antibody derived by fusion of Sp2/0 Mouse myeloma cells with spleen cells from a BALB/c Mouse immunized with a peptide comprising the first 10 amino acids of α-smooth muscle actin with an acetylated N-terminus coupled to keyhole limpet hemocyanin via the C-terminal cysteine (Ac-EEEDSTALVC).
Product
Each vial contains 50 ul 1 mg/ml purified monoclonal antibody in PBS containing 0.09% sodium azide.
Specificity
α-SM1 reacts exclusively with α-smooth muscle actin which is typical for vascular and visceral smooth muscle cells, but which is also present in myofibroblasts. The epitope that is recognized by α-SM1 is Ac-EEED.
Species Reactivity: The epitope recognized by α-SM1 is highly conserved. The antibody therefore cross-reacts with many species including protochordates, lower craniates and mammals.
Applications
α-SM1 is useful for immunohistochemistry on frozen and paraffin-embedded tissues, immunoblotting, immuno-electron microscopy and ELISA. Optimal antibody dilution should be determined by titration; recommended range is 1:100 – 1:250 for immunohistochemistry with avidin-biotinylated horseradish peroxidase complex (ABC) as detection reagent, and 1:100 – 1:500 for immunoblotting applications.
Storage
The antibody is shipped at ambient temperature and may be stored at +4°C. For prolonged storage prepare appropriate aliquots and store at or below -20°C. Prior to use, an aliquot is thawed slowly in the dark at ambient temperature, spun down again and used to prepare working dilutions by adding sterile phosphate buffered saline (PBS, pH 7.2). Repeated thawing and freezing should be avoided. Working dilutions should be stored at +4°C, not refrozen, and preferably used the same day. If a slight precipitation occurs upon storage, this should be removed by centrifugation. It will not affect the performance or the concentration of the product.
Shipping Conditions: Ship at ambient temperature.
Caution
This product is intended FOR RESEARCH USE ONLY, and FOR TESTS IN VITRO, not for use in diagnostic or therapeutic procedures involving humans or animals. It may contain hazardous ingredients. Please refer to the Safety Data Sheets (SDS) for additional information and proper handling procedures. Dispose product remainders according to local regulations.This datasheet is as accurate as reasonably achievable, but Nordic-MUbio accepts no liability for any inaccuracies or omissions in this information.
References
1. Skalli, O., Ropraz, P., Trzeciak, A., Benzonana, G., Gillessen, D. and Gabbiani, G. (1986). A monoclonal antibody anti alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103, 2787-96.
2. Skalli, O., Schurch, W., Seemayer, T., Lagace, R., Montandon, D., Pittet, B. and Gabbiani, G. (1989). Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest 60, 275-85.
3. Babaev, V.R., Bobryshev, Y.V., Stenina, O.V., Tararak, E.M. and Gabani, G. (1990). Heterogeneity of smooth muscle in atheromatous plaque of Human aorta. American Journal of Pathlogy 136, 1031-42.
4. Sappino, A. P., Schurch, W. and Gabbiani, G. (1990). Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 63, 144-61.
5. Vyalov, S. L., Gabbiani, G. and Kapanci, Y. (1993). Rat alveolar myofibroblasts acquire alpha-smooth muscle actin expression during bleomycin-induced pulmonary fibrosis. Am J Pathol 143, 1754-65.
6. Chaponnier, C., Goethals, M., Janmey, P. A., Gabbiani, F., Gabbiani, G. and Vandekerckhove, J. (1995). The specific NH2-terminal sequence Ac-EEED of alpha-smooth muscle actin plays a role in polymerization in vitro and in vivo. J Cell Biol 130, 887-95.
7. Simoncelli, F., Fagotti, A., Di Rosa, I., Panara, F., Chaponnier, C., Gabbiani, G. and Pascolini, R., (1996). Expression of an actin in protochordates and lower craniates defined by anti-aSM-1. European J of Cell Biol 69, 297-300.
8. Hinz, B., Celetta, G., Tomasek, J. J., Gabbiani, G. and Chaponnier, C. (2001). Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12, 2730-41.
9. Hinz, B., Gabbiani, G. and Chaponnier, C. (2002). The NH2-terminal peptide of alpha-smooth muscle actin inhibits force geneRation by the myofibroblast in vitro and in vivo. J Cell Biol 157, 657-63.
10. Hinz, B., Dugina, V., Ballestrem, C., Wehrle-Haller, B. and Chaponnier, C. (2003). Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell 14, 2508-19.
11. Clément, S., Hinz, B., Dugina, V., Gabbiani, G. and Chaponnier, C. (2004). The N-terminal Ac-EEED sequence plays a role in alpha-smooth-muscle actin incorporation into stress fibers. Journal of Cell Science 118, 1395-1404.
12. Chaponnier, C. and Gabbiani, G. (2004). Pathological situation characterized by altered actin isoform expression. J Pathol 204, 386-95.
13. Clément, S., Stouffs, M., Bettiol, E., Kampf, S., Krause, K., Chaponnier, C. and Jaconi, M. (2006). Expression and function of alpha-smooth muscle actin during embryonic-stem-cell-derived cardiomyocyte differentiation. Journal of Cell Science 120, 229-38.
14. De Visscher, G., Plusquin, R., Mesure, L. and Flameng, W. (2010). Selection of an immunohistochemical panel for cardiovascular research in Sheep. Appl Immunohistochem Mol Morphol 18, 382-91.
Protein Reference(s)
Database Name: UniProt
Accession Number: P62736
Safety Datasheet(s) for this product:
| NM_Sodium Azide |

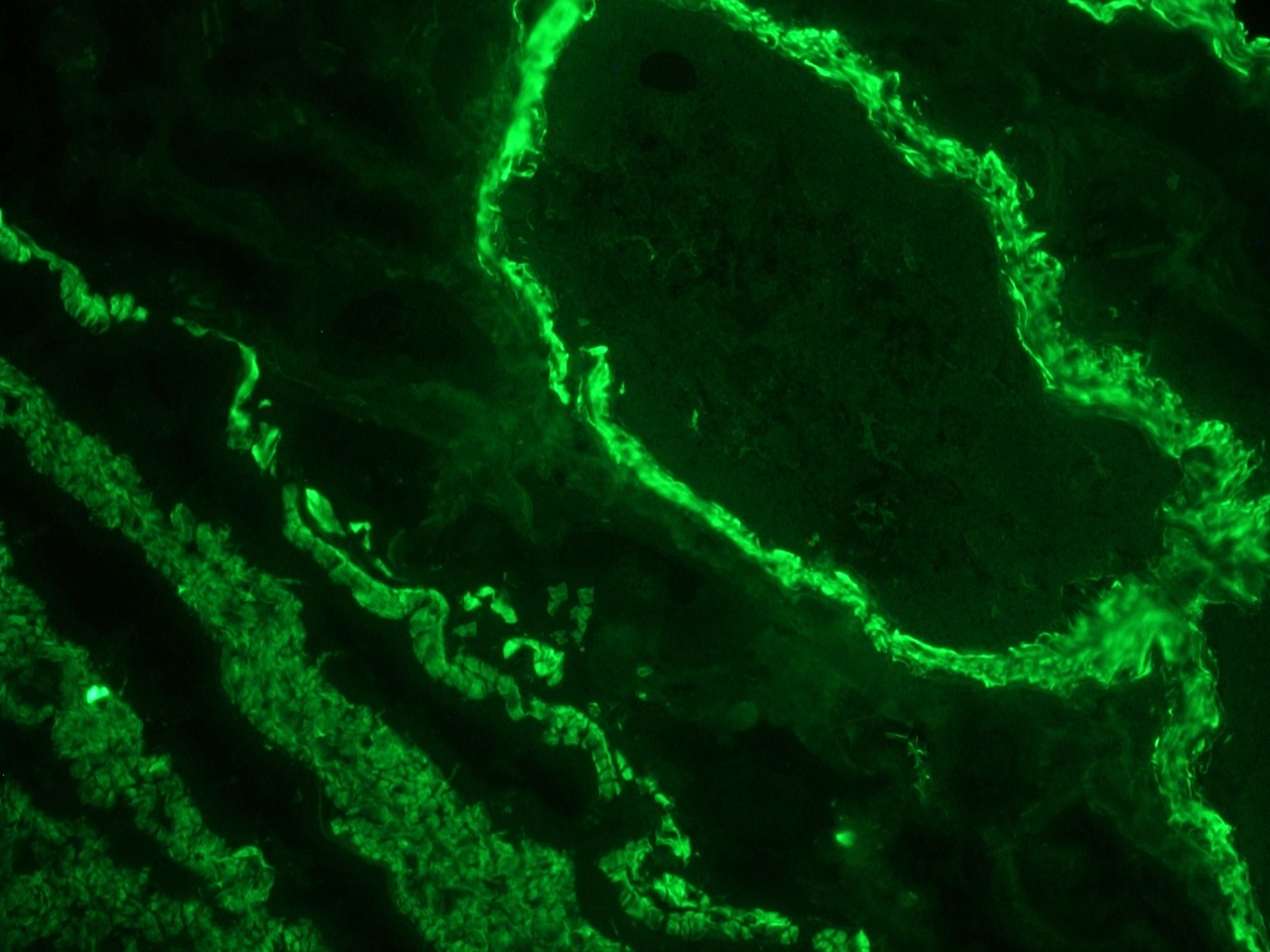
Figure 1. Indirect immunofluoresence staining of a frozen section of human colon using MUB0100P (clone 1A4) showing strong reactivity on the smooth muscle cells at a dilution of 1:500.

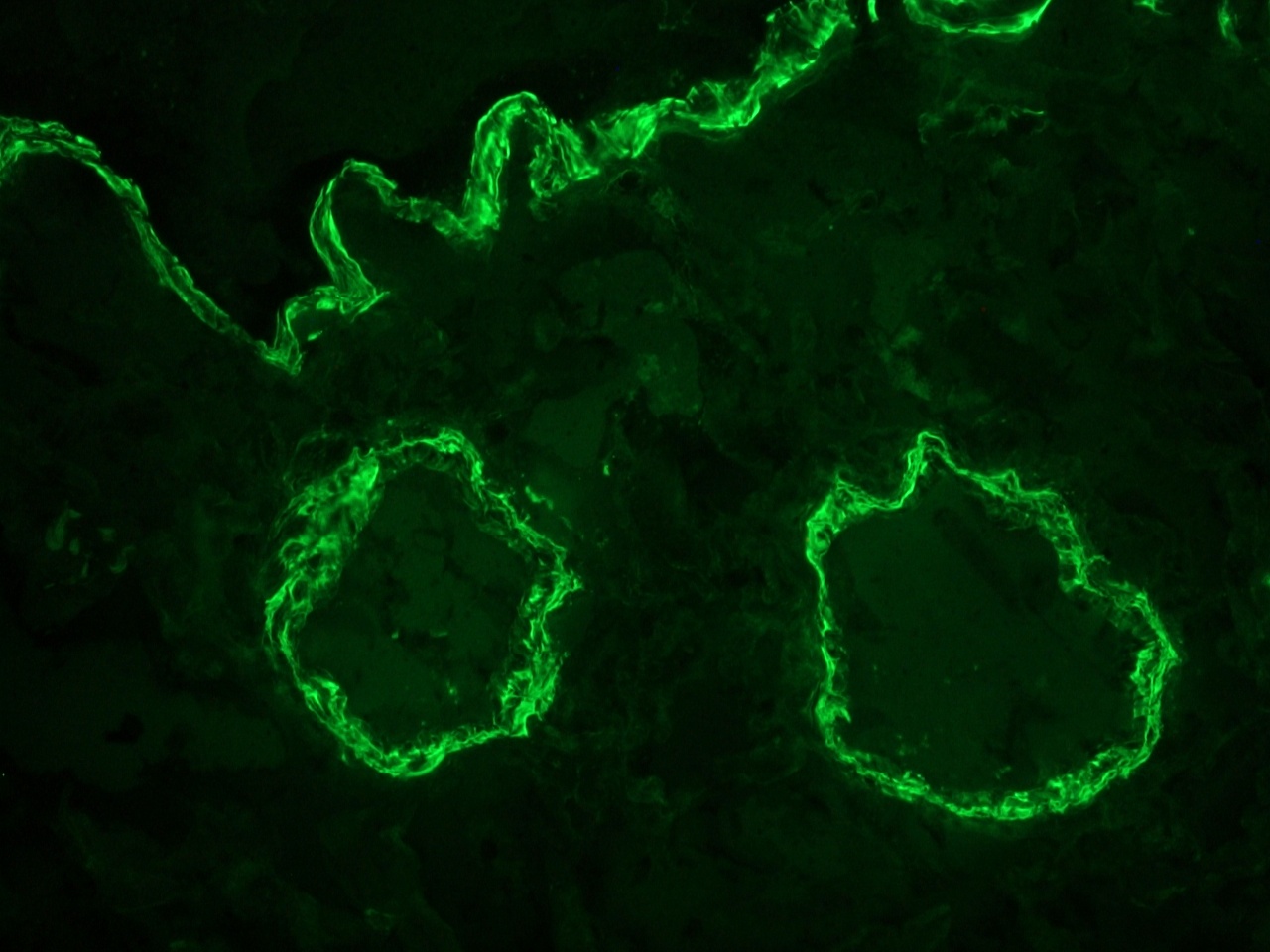
Figure 2. Indirect immunofluoresence staining of a frozen section of human colon using MUB0100P (clone 1A4) showing strong reactivity on the smooth muscle cells at a dilution of 1:500.