Catalogue
Mouse anti Lamin A
Catalog number: MUB1101P-CE/IVD| Clone | 133A2 |
| Isotype | IgG3 |
| Product Type |
Primary Antibodies |
| Units | 0.1 mg |
| Host | Mouse |
| Species Reactivity |
Bovine Canine Human Mouse Rat |
| Application |
Flow Cytometry Immunocytochemistry Immunohistochemistry (frozen) Immunohistochemistry (paraffin) Western Blotting |
Background
Nuclear lamins form a network of intermediate-type filaments at the nucleoplasmic site of the nuclear membrane. Two main subtypes of nuclear lamins can be distinguished, i.e. A-type lamins and B-type lamins. The A-type lamins comprise a set of three proteins arising from the same gene by alternative splicing, i.e. lamin A, lamin C and lamin Adel 10, while the B-type lamins include two proteins arising from two distinct genes, i.e. lamin B1 and lamin B2. Recent evidence has revealed that mutations in A-type lamins give rise to a range of rare but dominant genetic disorders, including Emery-Dreifuss muscular dystrophy, dilated cardiomyopathy with conduction-system disease and Dunnigan-type familial partial lipodystrophy. In addition, the expression of A-type lamins coincides with cell differentiation and as A-type lamins specifically interact with chromatin, a role in the regulation of differential gene expression has been suggested for A-type lamins.
Source
133A2 is a mouse monoclonal IgG3/κ antibody obtained from fusion of P3/X63.Ag8.653 mouse myeloma cells with spleen cells from a BALB/c mouse immunized with partially purified recombinant human lamin A.
Product
Each vial contains 100 ul 1 mg/ml purified monoclonal antibody in PBS containing 0.09% sodium azide. Since this IgG3 antibody has to be purified from the culture medium by ion-exchange chromatography it will contain remnants of bovine serum albumin.
Formulation: Each vial contains 100 ul 1 mg/ml purified monoclonal antibody in PBS containing 0.09% sodium azide. Since this IgG3 antibody has to be purified from the culture medium by ion-exchange chromatography it will contain remnants of bovine serum albumin.
Specificity
133A2 recognizes an epitope located between residues 598-611 of lamin A and therefore 133A2 reacts exclusively with lamin A.
Applications
133A2 is suitable for immunoblotting, immunocytochemistry on permeabilized cells, immunohistochemistry on frozen sections and paraffin embedded tissues, and flow cytometry. Optimal antibody dilution should be determined by titration; recommended range is 1:100 – 1:200 for immunocytochemistry, and immunohistochemistry with fluorescent secondary antibodies or avidin-biotinylated horseradish peroxidase complex (ABC) as detection reagent, and 1:100 – 1:1000 for immunoblotting applications.
Storage
The antibody is shipped at ambient temperature and may be stored at +4°C. For prolonged storage prepare appropriate aliquots and store at or below -20°C. Prior to use, an aliquot is thawed slowly in the dark at ambient temperature, spun down again and used to prepare working dilutions by adding sterile phosphate buffered saline (PBS, pH 7.2). Repeated thawing and freezing should be avoided. Working dilutions should be stored at +4°C, not refrozen, and preferably used the same day. If a slight precipitation occurs upon storage, this should be removed by centrifugation. It will not affect the performance or the concentration of the product.
Caution
When used for in vitro diagnostic purposes results must be put within the context of other diagnostic tests as well as the clinical history of the patient by a certified professional before final interpretation. Analyses performed with this antibody should be paralleled by positive and negative controls. If unexpected results are obtained which cannot be attributed to differences in laboratory procedures, please contact us. This product may contain hazardous ingredients. Please refer to the Safety Data Sheets (SDS) for additional information and proper handling procedures. Dispose product remainders according to local regulations.This datasheet is as accurate as reasonably achievable, but Nordic-MUbio accepts no liability for any inaccuracies or omissions in this information.
References
Recent application paper:
van Tienen FHJ, Lindsey PJ, Kamps MAF, Krapels IP, Ramaekers FCS, Brunner HG, van den Wijngaard A, Broers JLV. Assessment of fibroblast nuclear morphology aids interpretation of LMNA variants. Eur J Hum Genet. 2019 Mar;27(3):389-399. doi: 10.1038/s41431-018-0294-0.
1. Hozak, P., Sasseville, A. M., Raymond, Y., and Cook, P. R. (1995). Lamin proteins form an internal nucleoskeleton as well as a peripheral lamina in Human cells, J Cell Sci 108, 635-44.
2. Machiels, B. M., Broers, J. L., Raymond, Y., de Ley, L., Kuijpers, H. J., Caberg, N. E., and Ramaekers, F. C. (1995). Abnormal A-type lamin organization in a Human lung carcinoma cell line, Eur J Cell Biol 67, 328-35.
3. Broers, J. L., Machiels, B. M., Kuijpers, H. J., Smedts, F., van den Kieboom, R., Raymond, Y., and Ramaekers, F. C. (1997). A- and B-type lamins are differentially expressed in normal Human tissues, Histochem Cell Biol 107, 505-17.
4. Pugh, G. E., Coates, P. J., Lane, E. B., Raymond, Y., and Quinlan, R. A. (1997). Distinct nuclear assembly pathways for lamins A and C lead to their increase during quiescence in Swiss 3T3 cells, J Cell Sci 110, 2483-93.
5. Machiels, B. M., Ramaekers, F. C., Kuijpers, H. J., Groenewoud, J. S., Oosterhuis, J. W., and Looijenga, L. H. (1997). Nuclear lamin expression in normal testis and testicular germ cell tumours of adolescents and adults, J Pathol 182, 197-204.
6. Jansen, M. P., Machiels, B. M., Hopman, A. H., Broers, J. L., Bot, F. J., Arends, J. W., Ramaekers, F. C., and Schouten, H. C. (1997). Comparison of A and B-type lamin expression in reactive lymph nodes and nodular sclerosing Hodgkin's disease, Histopathology 31, 304-12.
7. Neri, L. M., Raymond, Y., Giordano, A., Borgatti, P., Marchisio, M., Capitani, S., and Martelli, A. M. (1999). Spatial distribution of lamin A and B1 in the K562 cell nuclear matrix stabilized with metal ions, J Cell Biochem 75, 36-45.
8. Neri, L. M., Raymond, Y., Giordano, A., Capitani, S., and Martelli, A. M. (1999). Lamin A is part of the internal nucleoskeleton of Human erythroleukemia cells, J Cell Physiol 178, 284-95.
9. Broers, J. L., Machiels, B. M., van Eys, G. J., Kuijpers, H. J., Manders, E. M., van Driel, R., and Ramaekers, F. C. (1999). Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins, J Cell Sci 112, 3463-75.
10. Broers, J. L., Bronnenberg, N. M., Kuijpers, H. J., Schutte, B., Hutchison, C. J., and Ramaekers, F. C. (2002). Partial cleavage of A-type lamins concurs with their total disintegration from the nuclear lamina during apoptosis. Eur J Cell Biol 81, 677-691.
11. De Sandre-Giovannoli, A., Bernard, R., Cau, P., Navarro, C., Amiel, J., Boccaccio, I., Lyonnet, S., Stewart, C. L., Munnich, A., Le Merrer, M., and Levy, N. (2003). Lamin a trunCation in Hutchinson-Gilford progeria. Science 300, 2055.
12. Eriksson, M., Brown, W. T., Gordon, L. B., Glynn, M. W., Singer, J., Scott, L., Erdos, M. R., Robbins, C. M., Moses, T. Y., Berglund, P., et al. (2003). Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293-298.
CE Mark
CE
Safety Datasheet(s) for this product:
| NM_Sodium Azide |

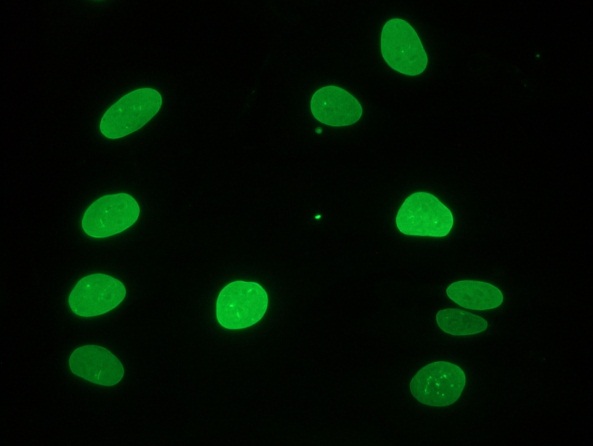
Figure 1 Immunocytochemical staining of fiboblasts showing nuclear lamina

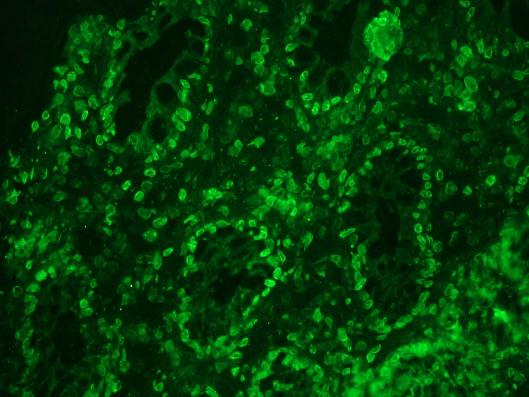
Figure 2 Immunohistochemistry on frozen sections of human colon showing nuclear lamina staining in epithelial and connective tissue cells

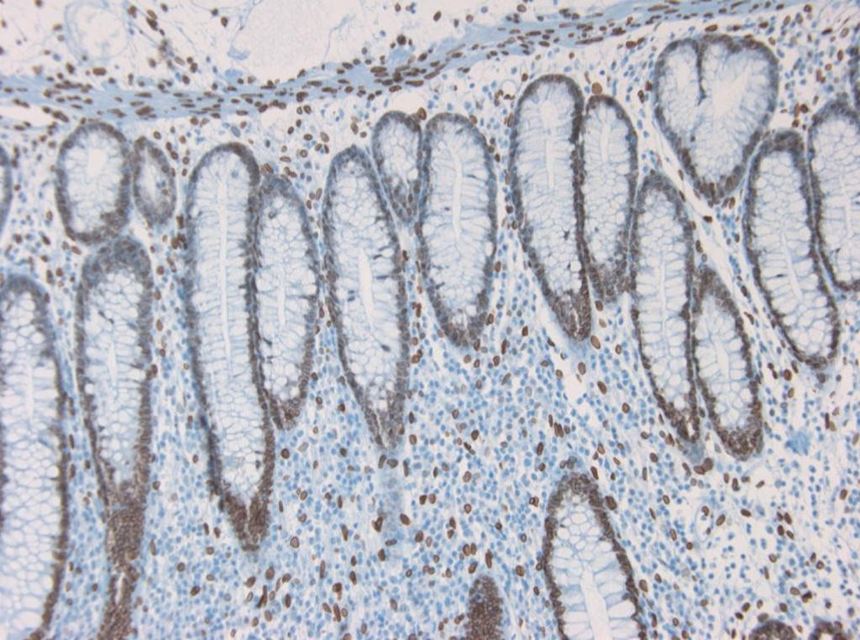
Figure 3 Immunohistochemistry on paraffin section of human colon