Catalogue
Mouse anti Cytokeratin 18 / Keratin K18
Catalog number: MUB0326P-CE/IVD| Clone | RGE53 |
| Isotype | IgG1 |
| Product Type |
Primary Antibodies |
| Units | 0.1 mg |
| Host | Mouse |
| Species Reactivity |
Canine Chicken Hamster Human Mouse Rabbit Rat Swine Zebrafish |
| Application |
Flow Cytometry Immunocytochemistry Immunohistochemistry (frozen) Western Blotting |
Background
Cytokeratins are a subfamily of intermediate filament proteins and are characterized by a remarkable biochemical diversity, represented in Human epithelial tissues by at least 20 different polypeptides. They range in molecular weight between 40 kDa and 68 kDa and isoelectric pH between 4.9 – 7.8. The individual Human Cytokeratins are numbered 1 to 20. The various epithelia in the Human body usually express Cytokeratins which are not only characteristic of the type of epithelium, but also related to the degree of matuRation or differentiation within an epithelium. Cytokeratin subtype expression patterns are used to an increasing extent in the distinction of different types of epithelial malignancies. The Cytokeratin antibodies are not only of assistance in the differential diagnosis of tumors using immunohistochemistry on tissue sections, but are also a useful tool in cytopathology and flow cytometric assays.
Source
RGE53 is a Mouse monoclonal IgG1 antibody derived by fusion of SP2/0-Ag14 mouse myeloma cells with spleen cells from a BALB/c mouse immunized with a cytoskeletal preparation of HeLa cells.
Product
Each vial contains 100 ul 1 mg/ml purified monoclonal antibody in PBS containing 0.09% sodium azide.
Formulation: Each vial contains 100 ul 1 mg/ml purified monoclonal antibody in PBS containing 0.09% sodium azide.
Specificity
RGE53 reacts exclusively with Cytokeratin 18 which is present in glandular epithelial cells of the digestive, respiRatory, and urogenital tracts, endocrine and exocrine cells and mesothelial cells, as well as adenocarcinomas originating from them.
Applications
RGE53 is suitable for immunoblotting, immunocytochemistry on acetone fixed cells, immunohistochemistry on frozen sections and flow cytometry. Optimal antibody dilution should be determined by titration; recommended range is 1:100 – 1:200 for immunohistochemistry with avidin-biotinylated Horseradish peroxidase complex (ABC) as detection reagent, and 1:100 – 1:1000 for immunoblotting applications.
Storage
The antibody is shipped at ambient temperature and may be stored at +4°C. For prolonged storage prepare appropriate aliquots and store at or below -20°C. Prior to use, an aliquot is thawed slowly in the dark at ambient temperature, spun down again and used to prepare working dilutions by adding sterile phosphate buffered saline (PBS, pH 7.2). Repeated thawing and freezing should be avoided. Working dilutions should be stored at +4°C, not refrozen, and preferably used the same day. If a slight precipitation occurs upon storage, this should be removed by centrifugation. It will not affect the performance or the concentration of the product.
Caution
When used for in vitro diagnostic purposes results must be put within the context of other diagnostic tests as well as the clinical history of the patient by a certified professional before final interpretation. Analyses performed with this antibody should be paralleled by positive and negative controls. If unexpected results are obtained which cannot be attributed to differences in laboratory procedures, please contact us. This product may contain hazardous ingredients. Please refer to the Safety Data Sheets (SDS) for additional information and proper handling procedures. Dispose product remainders according to local regulations.This datasheet is as accurate as reasonably achievable, but Nordic-MUbio accepts no liability for any inaccuracies or omissions in this information.
References
1. Ramaekers, F., Huysmans, A., Moesker, O., Kant, A., Jap, P., Herman, C., and Vooijs, P. (1983). Monoclonal antibody to Keratin filaments, specific for glandular epithelia and their tumors. Use in surgical pathology, Lab Invest 49, 353-61.
2. Ramaekers, F. C., Puts, J. J., Moesker, O., Kant, A., Huysmans, A., Haag, D., Jap, P. H., Herman, C. J., and Vooijs, G. P. (1983). Antibodies to intermediate filament proteins in the immunohistochemical identifiCation of Human tumours: an overview, Histochem J 15, 691-713.
3. Puts, J. J., Moesker, O., Kenemans, P., Vooijs, G. P., and Ramaekers, F. C. (1985). Expression of Cytokeratins in early neoplastic epithelial lesions of the uterine cervix, Int J Gynecol Pathol 4, 300-13.
4. Ramaekers, F., van Niekerk, C., Poels, L., Schaafsma, E., Huijsmans, A., Robben, H., Schaart, G., and Vooijs, P. (1990). Use of monoclonal antibodies to Keratin 7 in the differential diagnosis of adenocarcinomas, Am J Pathol 136, 641-55.
5. Raats, J. M., Pieper, F. R., Vree Egberts, W. T., Verrijp, K. N., Ramaekers, F. C., and Bloemendal, H. (1990). Assembly of amino-terminally deleted desmin in vimentin-free cells, J Cell Biol 111, 1971-85.
6. Smedts, F., Ramaekers, F., Robben, H., Pruszczynski, M., van Muijen, G., Lane, B., Leigh, I., and Vooijs, P. (1990). Changing patterns of Keratin expression during progression of cervical intraepithelial neoplasia, Am J Pathol 136, 657-68.
7. Smedts, F., Ramaekers, F., Troyanovsky, S., Pruszczynski, M., Link, M., Lane, B., Leigh, I., Schijf, C., and Vooijs, P. (1992). Keratin expression in cervical cancer, Am J Pathol 141, 497-511.
8. van Leenders, G., Dijkman, H., Hulsbergen-van de Kaa, C., Ruiter, D., and Schalken, J. (2000). Demonstration of intermediate cells during Human prostate epithelial differentiation in situ and in vitro using triple-staining confocal scanning microscopy, Lab Invest 80, 1251-8.
CE Mark
CE
Protein Reference(s)
Database Name: UniProt
Accession Number: P05783
Safety Datasheet(s) for this product:
| NM_Sodium Azide |

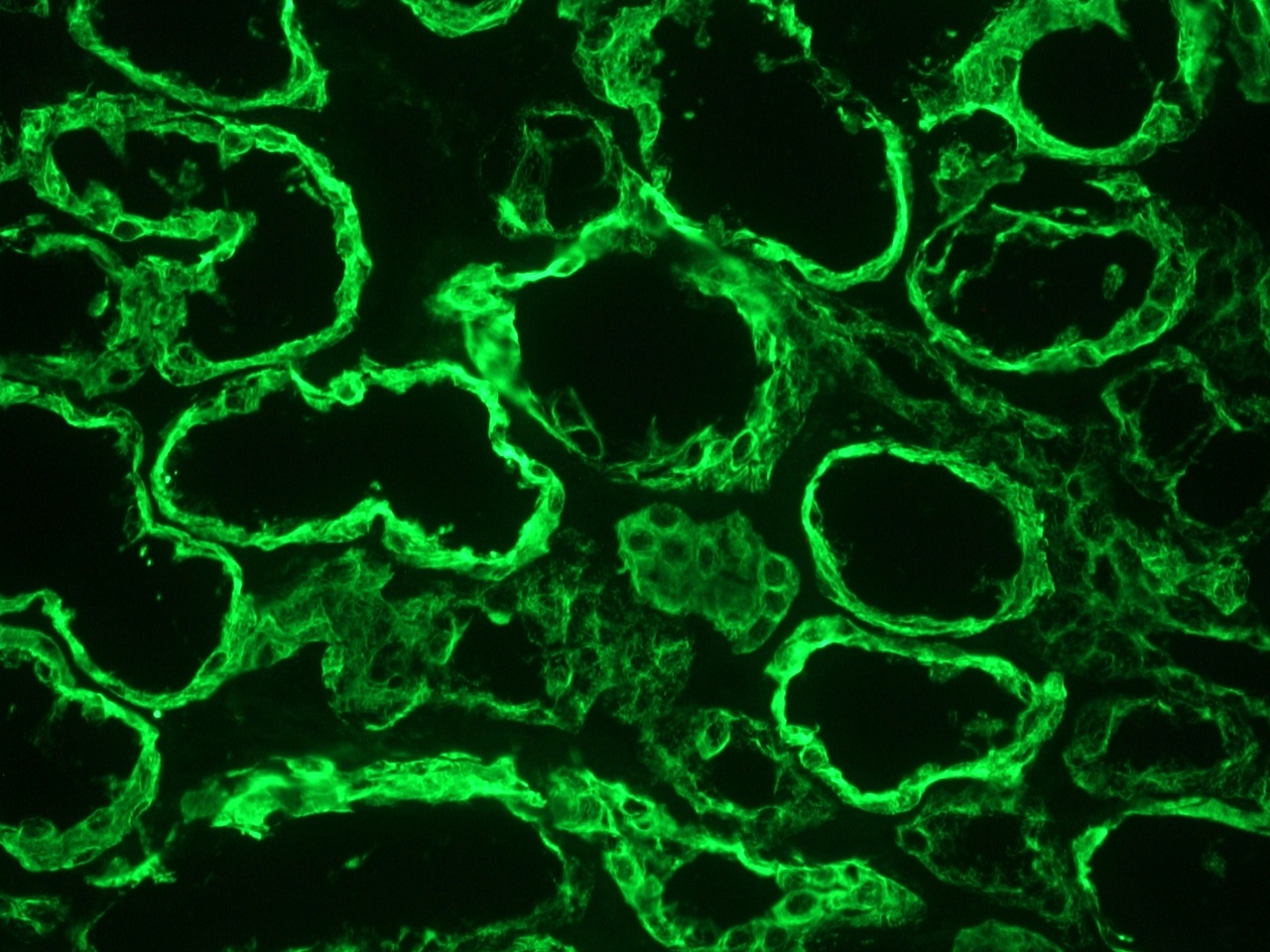
Figure 1. MUB0326P Immunohistochemistry on frozen section of human kidney epithelium.

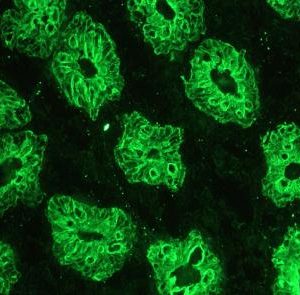
Figure 2. MUB0326P immunohistochemistry on frozen section of human colon.

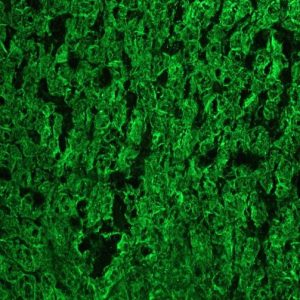
Figure 3. MUB0326P immunohistochemistry on frozen section of swine liver hepatocytes.

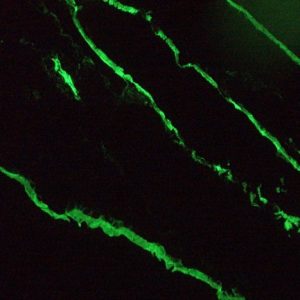
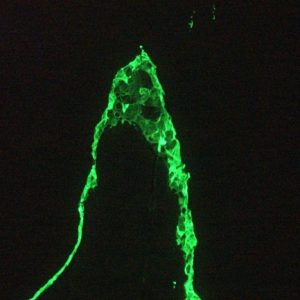
Figure 4. MUB0326P immunofluorescence staining of epithelial tissues in a 2 days old zebrafish embryo.

Figure 5. MJB0326P immunofluorescence staining of 1 month old zebrafish embryo.

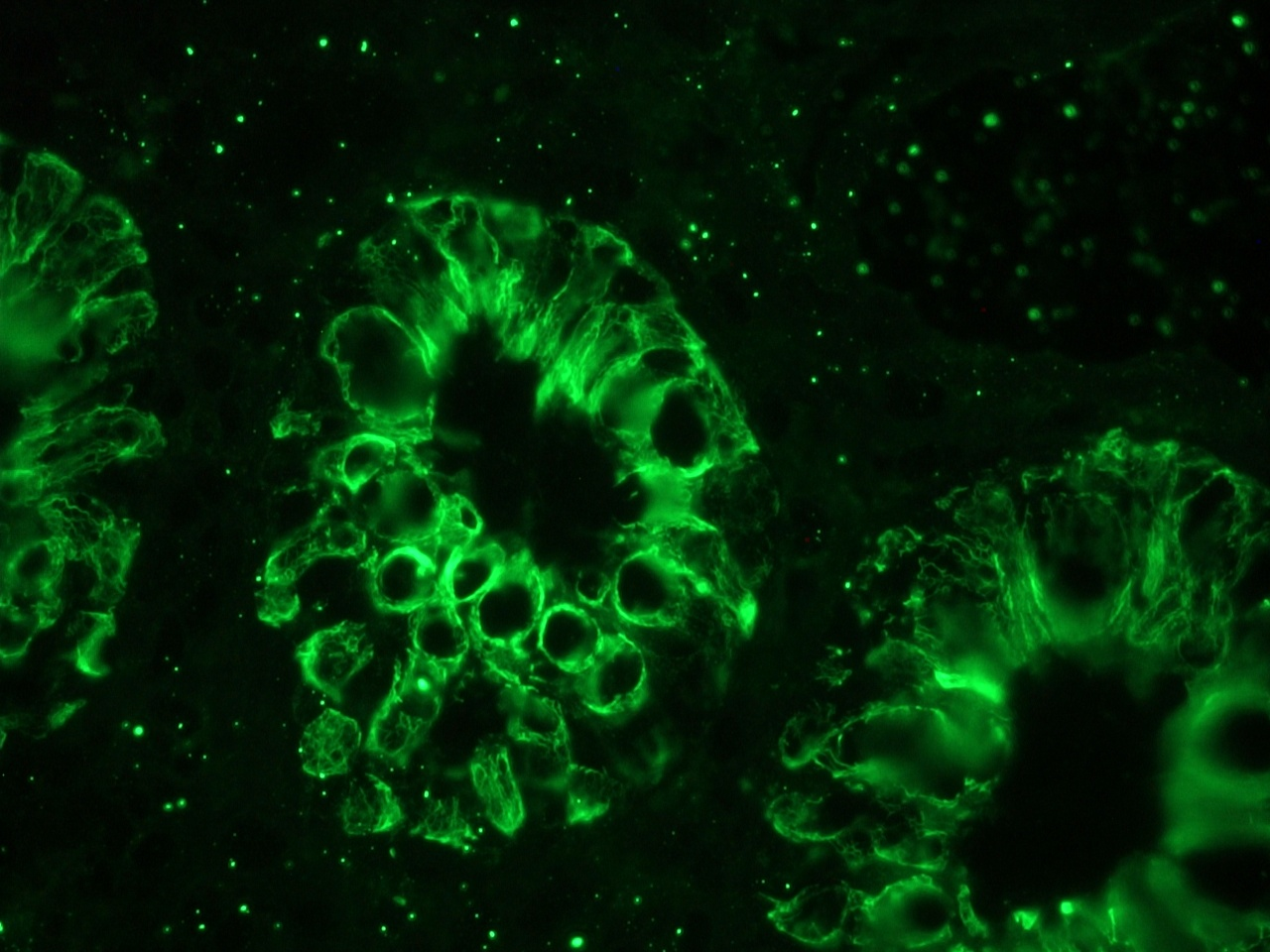
Figure 6. MUB0326P immunofluorescence staining of human colon epithelium.